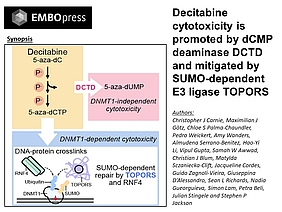
New joint publication by the Beli and Stingele labs on how decitabine cytotoxicity is promoted by dCMP deaminase DCTD and mitigated by SUMO-dependent E3 ligase TOPORS
Carnie C, Götz M, Palma-Chaundler C, Weickert P, Wanders A, Serrano-Benitez A, Li H, Gupta V, Awwad S, Blum C, Sczaniecka-Clift M, Cordes J, Zagnoli-Vieira G, D´Alessandro G, Richards S, Gueorguieva N, Lam S, Beli P, Stingele J, Jackson S (2024) Decitabine cytotoxicity is promoted by dCMP deaminase DCTD and mitigated by SUMO-dependent E3 ligase TOPORS. EMBO J., doi: 10.1038/s44318-024-00108-2
Abstract:
The nucleoside analogue decitabine (or 5-aza-dC) is used to treat several haematological cancers. Upon its triphosphorylation and incorporation into DNA, 5-aza-dC induces covalent DNA methyltransferase 1 DNA–protein crosslinks (DNMT1-DPCs), leading to DNA hypomethylation. However, 5-aza-dC’s clinical outcomes vary, and relapse is common. Using genome-scale CRISPR/Cas9 screens, we map factors determining 5-aza-dC sensitivity. Unexpectedly, we find that loss of the dCMP deaminase DCTD causes 5-aza-dC resistance, suggesting that 5-aza-dUMP generation is cytotoxic. Combining results from a subsequent genetic screen in DCTD-deficient cells with the identification of the DNMT1-DPC-proximal proteome, we uncover the ubiquitin and SUMO1 E3 ligase, TOPORS, as a new DPC repair factor. TOPORS is recruited to SUMOylated DNMT1-DPCs and promotes their degradation. Our study suggests that 5-aza-dC-induced DPCs cause cytotoxicity when DPC repair is compromised, while cytotoxicity in wild-type cells arises from perturbed nucleotide metabolism, potentially laying the foundations for future identification of predictive biomarkers for decitabine treatment.
Read the full paper here: https://www.embopress.org/doi/full/10.1038/s44318-024-00108-2